oomnia : Comprehensive software solution designed for clinical trials

oomnia: in summary
oomnia by Wemedoo is the first true all-in-one clinical trial solution, unifying clinical research and clinical practice within a single platform. It integrates all essential clinical trial tools—including EDC, RTSM, CTMS, eTMF, ePRO, eCOA, eConsent, and eSource—into one seamless system. Designed for hospitals, research organizations, and clinical trial sponsors, Oomnia improves efficiency, reduces errors, and ensures regulatory compliance while enabling real-time oversight and collaboration across clinical workflows.
Key benefits include up to 80% time savings, improved data quality, enhanced patient care, and a foundation for future AI-driven healthcare modules.
What are the main features of Oomnia?
Unified clinical trial platform
Oomnia consolidates all core trial components into one system:
EDC (Electronic Data Capture) for accurate, real-time data collection
RTSM (Randomization and Trial Supply Management) to ensure smooth trial operations
CTMS (Clinical Trial Management System) to track study progress and milestones
eTMF (Electronic Trial Master File) for centralized, compliant documentation
ePRO/eCOA (Patient-Reported and Clinical Outcome Assessments) to gather patient data digitally
eConsent and eSource for streamlined patient enrollment and source data collection
Real-time collaboration and data interoperability
Oomnia ensures full clinical data interoperability, connecting seamlessly with any EHR or existing clinical system:
Instant access to trial and clinical practice data
Real-time collaboration between investigators, coordinators, and sponsors
Integration without programming or custom development
Supports trial-agnostic workflows adaptable to specific study requirements
Workflow automation and efficiency
Oomnia reduces manual work and errors, significantly accelerating trial processes:
Streamlined data entry, validation, and reporting
Automation of routine tasks, reducing administrative overhead
Continuous oversight of trial and clinical workflows
Up to 80% time savings on repetitive processes
Clinical data quality and regulatory compliance
Oomnia safeguards data integrity and compliance:
Compliance with industry standards and regulations
Audit-ready records with full traceability
Standardized processes to improve patient care and trial outcomes
Foundation for future AI integration
Oomnia’s clinical core is AI-ready, ensuring compatibility with next-generation healthcare modules:
Structured, high-quality datasets
Scalable architecture for AI-driven insights
Continuous support for innovation in clinical research
Why choose Oomnia?
All-in-one platform: Combines research and clinical practice tools in a single system
Trial-agnostic and adaptable: Works with any EHR and flexible workflows
Time and labor savings: Up to 80% reduction in manual tasks
Real-time oversight: Full visibility across trials and clinical operations
AI-ready clinical core: Future-proof solution for emerging healthcare technologies
Oomnia streamlines clinical trial management, reduces operational complexity, and enhances patient outcomes by unifying research and practice in one scalable, AI-ready platform.
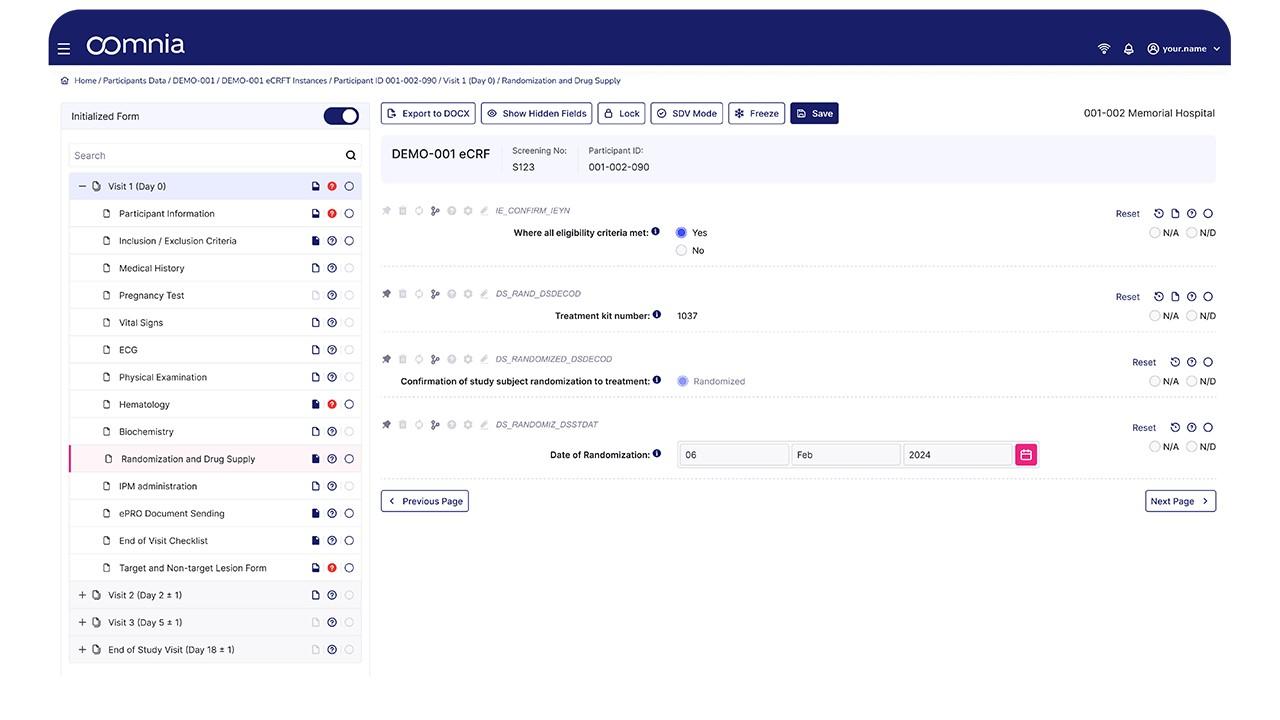 oomnia - Screenshot 1
oomnia - Screenshot 1 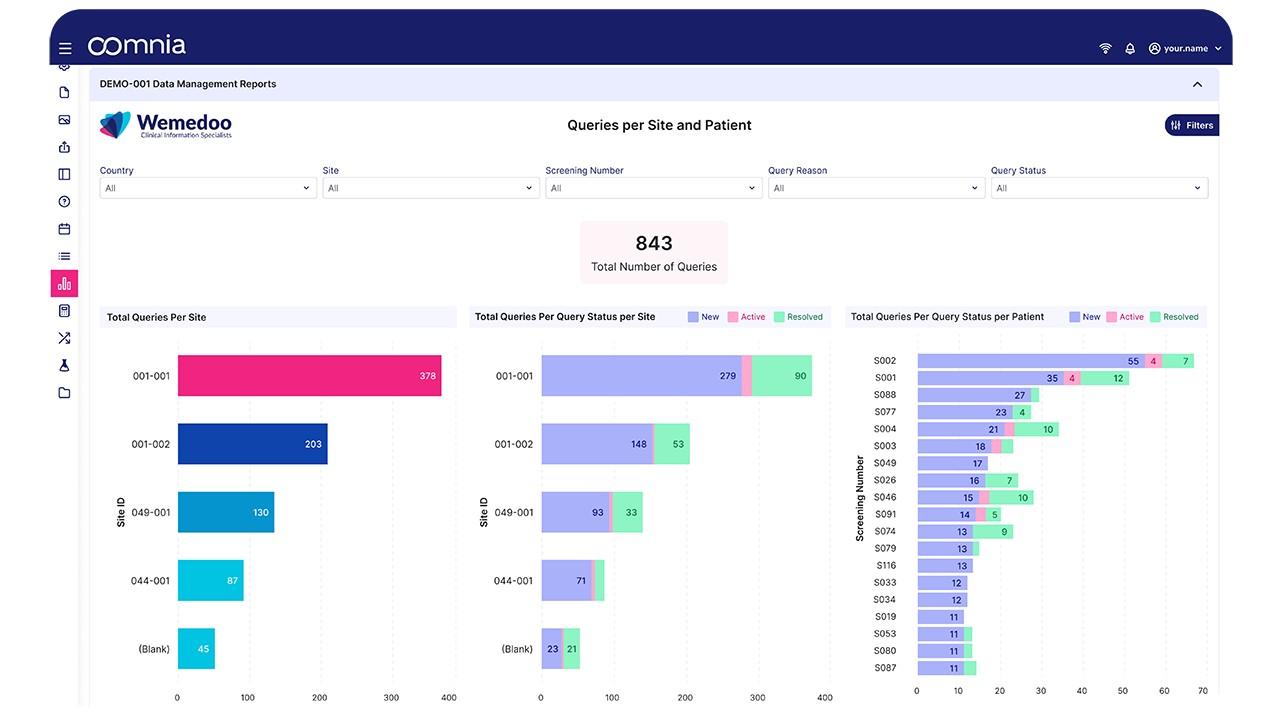 oomnia - Screenshot 2
oomnia - Screenshot 2 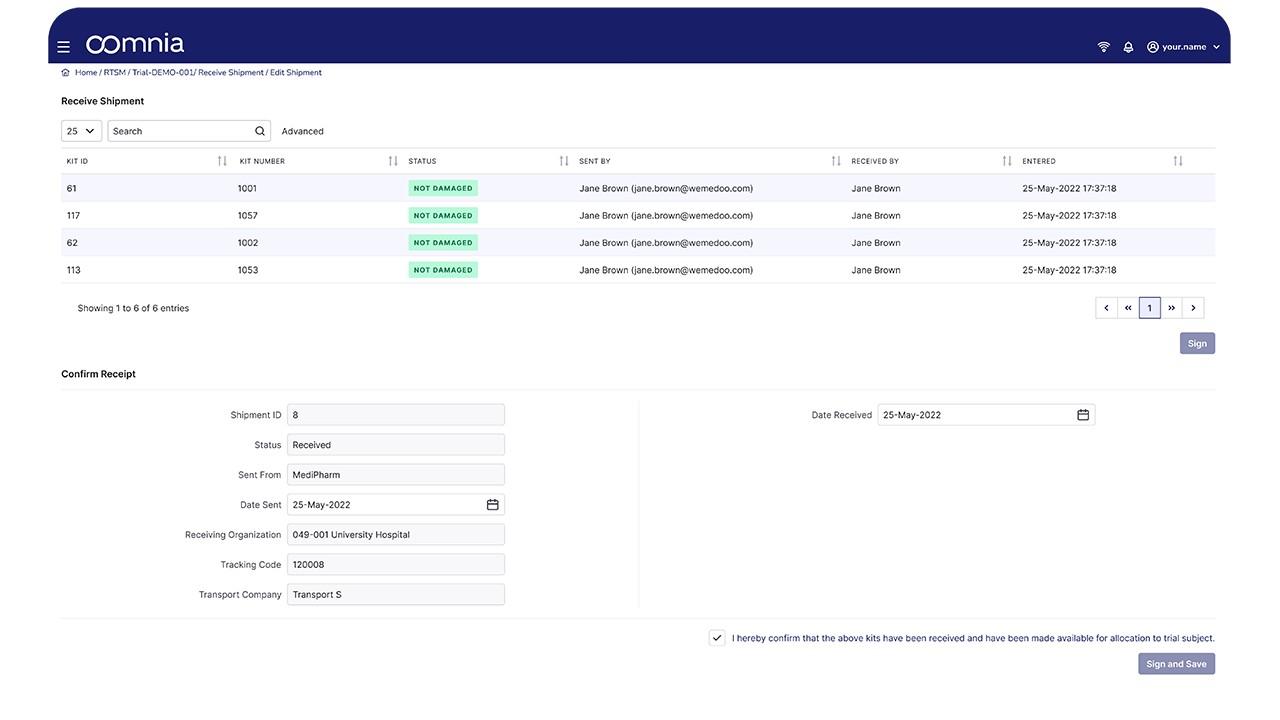 oomnia - Screenshot 3
oomnia - Screenshot 3 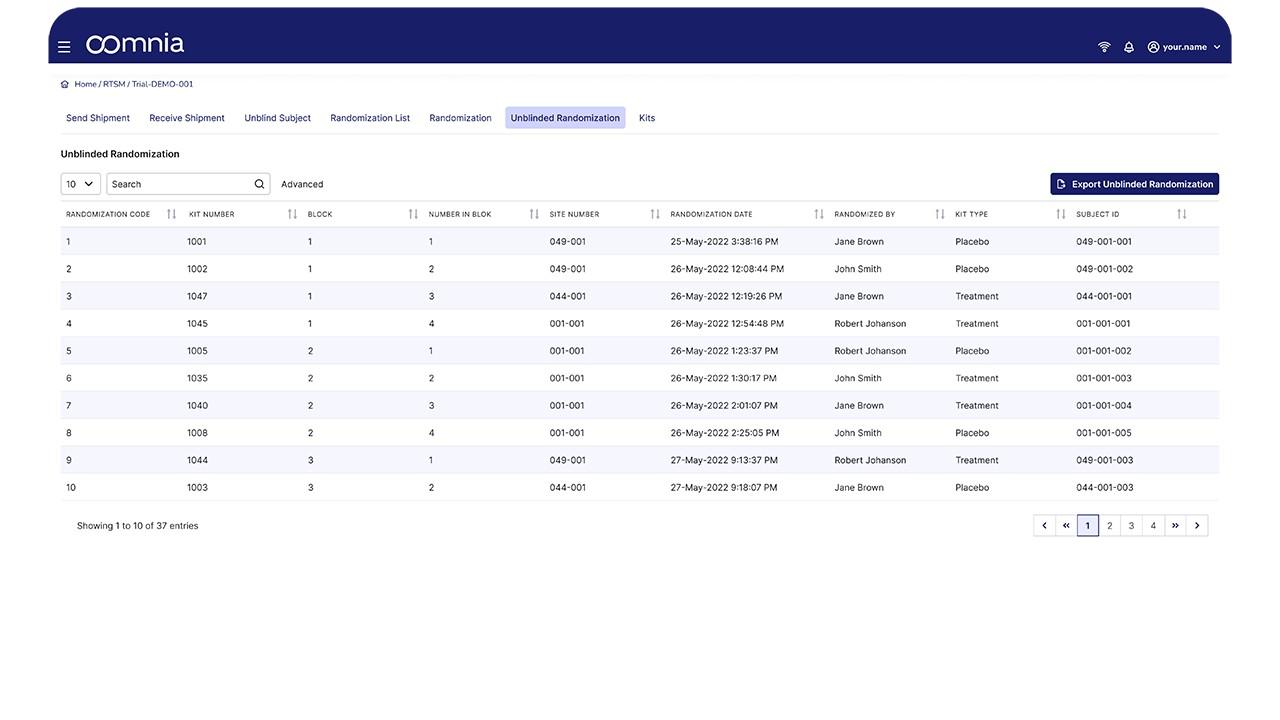 oomnia - Screenshot 4
oomnia - Screenshot 4 



oomnia: its rates
standard
Rate
On demand
Clients alternatives to oomnia

Streamline clinical trials with our software. Manage participants, track data, and create reports all in one place.
See more details See less details
Our Clinical Trial Management software is designed to simplify the entire process. With i-OMS, you can easily schedule visits, monitor participant progress, and securely store data. Plus, our reporting tools make it easy to share results with stakeholders.
Read our analysis about i-OMSTo i-OMS product page

Streamline clinical trials with our software. Manage data, track progress, and automate processes to save time and reduce errors.
See more details See less details
Our Clinical Trial Management software simplifies trial management. With real-time data access, customisable reporting, and secure data storage, you can ensure compliance and streamline communication between team members.
Read our analysis about InclinicalPerformTo InclinicalPerform product page

A clinical trial management software that streamlines trial processes, from planning to execution. Enables real-time tracking of progress, data management, and collaboration.
See more details See less details
With MATRIX CTMS, users can easily manage patient data, track inventory, and monitor finances. The software also supports regulatory compliance and provides customisable reporting. Its intuitive interface and automated workflows make it easy to use.
Read our analysis about MATRIX CTMSTo MATRIX CTMS product page
Appvizer Community Reviews (0) The reviews left on Appvizer are verified by our team to ensure the authenticity of their submitters.
Write a review No reviews, be the first to submit yours.
